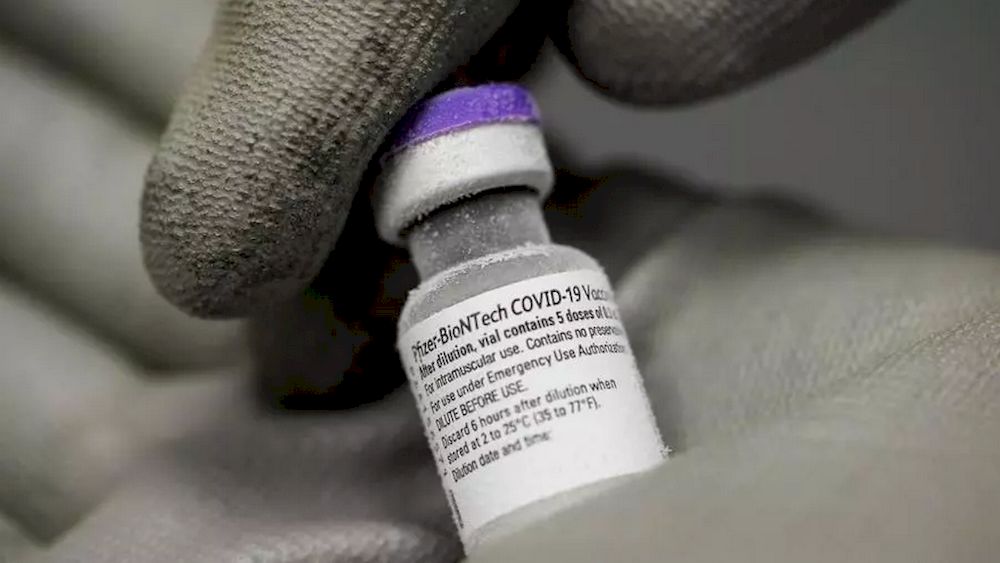
KUALA LUMPUR, Feb 20 — Arriving in Malaysia tomorrow is the country’s first batch of Covid-19 vaccines — 312,390 doses of the Pfizer-BioNTech vaccine, Comirnaty, ahead of the National Covid-19 Immunisation Programme that is scheduled to begin on February 26.
As the movement of the incoming vaccine is still under wraps, lets re-look at what Pfizer had said about the process of distribution to the point of use (POU) as the vaccine needs to be stored frozen after manufacture.
According to the pharmaceutical company, it will ship its vaccines largely from its Kalamazoo (Michigan, United States) and Puurs (Belgium) sites direct to the POU. Pfizer will also be using its existing distribution centres in Wisconsin, United States, and Karlsruhe, Germany.
Pfizer chairman and CEO Dr Albert Bourla, in an open letter dated December 15, said the company’s engineers created specially designed, temperature-controlled shipping containers or “shippers” which contain GPS temperature-enabled trackers for continuous, real-time location and temperature monitoring.
The shippers can maintain the required temperature conditions for up to 10 days unopened, making it possible to maintain vaccine integrity as these containers travel across the globe to the POU.
Pfizer control towers monitor and track all shipments round the clock to ensure each shipment gets to where it needs to go at the right time and at the right temperature and take action, if needed, along the way.
Once the shippers arrive at the POU, there are three options for storage as stated on the Pfizer website including using ultra-low-temperature freezers which can extend shelf life for up to six months.
Secondly, the Pfizer thermal shippers can also be used as temporary storage units by refilling with dry ice for up to 15 days of storage. The shippers can maintain temperature for 10 days unopened. Once opened, and if being used as temporary storage by a vaccination centre, it can then be used for a total of 15 days with re-icing every five days.
Thirdly, by using refrigeration units that are commonly available in hospitals. The vaccine can be stored for five days at refrigerated 2-8 degrees Celsius conditions. Once thawed and stored under 2-8 degrees Celsius conditions, the vials cannot be re-frozen or stored under frozen conditions, it said.
Pfizer-BioNTech Friday announced the submission of new data to the US Food and Drug Administration demonstrating the stability of its vaccine when stored at -25 to -15 degrees Celsius, temperatures more commonly found in pharmaceutical freezers and refrigerators.
Currently, the labels for the Pfizer-BioNTech Covid-19 vaccine state that they must be stored in an ultra-cold freezer at temperatures of between -80 and -60 degrees Celsius.
The vaccine, jointly developed by US pharmaceutical giant Pfizer and BioNTech, a biotechnology company founded by Turkish-German couple Dr Ugur Sahin and his wife Dr Ozlem Tureci, has an efficacy rate of 95 per cent after two doses.
According to reports, among countries that have administered the vaccine are the United States, the United Kingdom, countries of the European Union, Canada, Costa Rica, Kuwait, Brazil and Singapore.
On January 11, an agreement for the delivery of the first phase of the Covid-19 Pfizer-BioNTech was signed between the Malaysian government, through the Health Ministry (MOH), and Pfizer (Malaysia) Sdn Bhd.
The agreement involves the procurement of 12,799,800 doses of vaccine, covering 20 per cent of Malaysian population, with two doses per person.
The MOH is in talks with Pfizer to obtain another 30 per cent supply of the Covid-19 vaccine that would cover 50 per cent of population.
As of this month, Malaysia had secured access to 66.7 million doses of Covid-19 vaccine through Covax Facility from five producers, namely Pfizer-BioNTech, AstraZeneca, sinovac, CanSinoBIO and Sputnik V. — Bernama